
Medical Device Prototyping
Innovative solutions in aerospace manufacturing ensuring quality and efficiency.
Navigating the Complex World of Medical Device Prototyping with Avalon Technologies
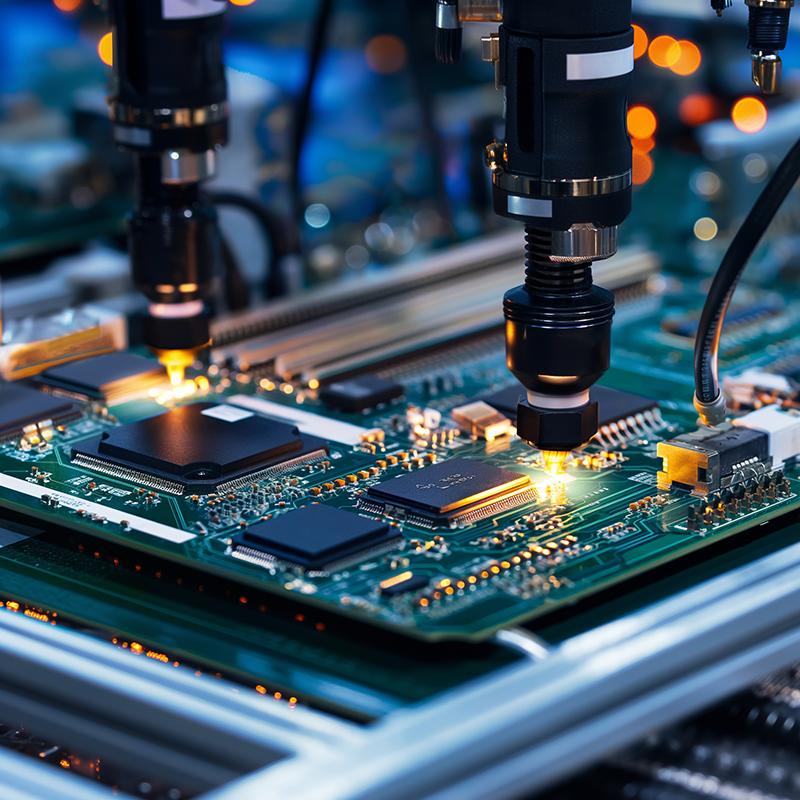
In the dynamic field of medical technology, the creation of medical electronics is both a critical and intricate process. As medical devices play a crucial role in patient care, the prototyping stage is particularly vital. This is where Avalon Technologies, a leader in electronic manufacturing and IoT solutions in India, steps in to guide the development from concept to market-ready innovations.
Cutting-Edge Medical Device Prototyping Techniques
Medical devices are more than just complex electronics, they are essential to life-saving treatments and diagnostics. Even the smallest error can have significant repercussions, which is why precision is paramount at every step of the prototyping process.
At Avalon Technologies, we employ state-of-the-art technology and deep medical expertise to ensure each prototype is developed with the utmost reliability and efficiency.
Regulatory Compliance and Safety
The medical field is one of the most heavily regulated industries globally, necessitating strict adherence to numerous standards and regulations. Avalon Technologies understanding of these regulatory environments is very thorough and are committed to meet the rigorous standards.
Collaborative Prototyping
Developing medical electronics requires a comprehensive approach that benefits greatly from collaboration. Avalon Technologies engages closely with healthcare professionals and industry stakeholders to align the device’s functionality with real-world medical needs, thereby enhancing both its usability and technological sophistication.
This partnership facilitates a quicker prototyping process and improves the overall design and user experience of the final product.
Scalability and Market Introduction
Addressing the scalability of a medical device early in the prototyping phase is crucial for a smooth transition to mass production. Avalon Technologies’ in-depth knowledge of manufacturing processes ensures that each prototype is designed with scalability in focus, which minimizes the time-to-market and supports a seamless shift from prototype to production.
Risk Mitigation and Quality Assurance
With high stakes in the medical electronics field, it’s essential to take measured precautions to mitigate risks. Avalon Technologies achieves this by
- Rigorously validating prototype design and functionality to confirm they meet specific use cases and maintain patient safety.
- Conducting extensive testing and quality checks to address any potential failures or compliance issues promptly.
- Staying updated with the latest advancements and regulatory shifts in medical technology to ensure continued relevance and compliance through the development cycle.
Summary
Avalon Technologies stands out as a beacon of innovation and reliability in the medical electronics sector. With a strong emphasis on technological sophistication, regulatory compliance, and collaborative development.
Avalon is ideally positioned to navigate the complexities of medical device prototyping, ensuring that each prototype not only leads to innovative medical solutions but also adheres to the highest standards of patient safety and care.
FAQs About Contract Manufacturing
Contact Us






















